
eCRF – clinical
trial software
Safety and credibility of clinical trials.
Non-commercial clinical trials
Commercial clinical trials
Research
Compliant with FDA standards

Non-commercial clinical trials
Commercial clinical trials
Research
Compliant with FDA standards

Integrated with the hospital system
Flexible, adapts to changes in the test protocol
Configurable for any protocol
Quick to implement
Intuitive to use
Doesn't require extra training
Our eCRF solution was created on the basis of many years of experience of researchers and medical teams with whom we carried out research projects. The system is, above all, easy to use, user-friendly and extremely flexible.
The prepared solution meets all the required quality standards, and thanks to the full control over the code, we can further develop and adapt it to the changing requirements of regulators and the clinical trials market.
Our eCRF supports the process of designing and managing multiple clinical trials. In each study, a view of the full list of patients is available, showing the stages they are currently in with the possibility of managing a given patient in the study (randomization, dispensing / returning of test products, management of patient samples).
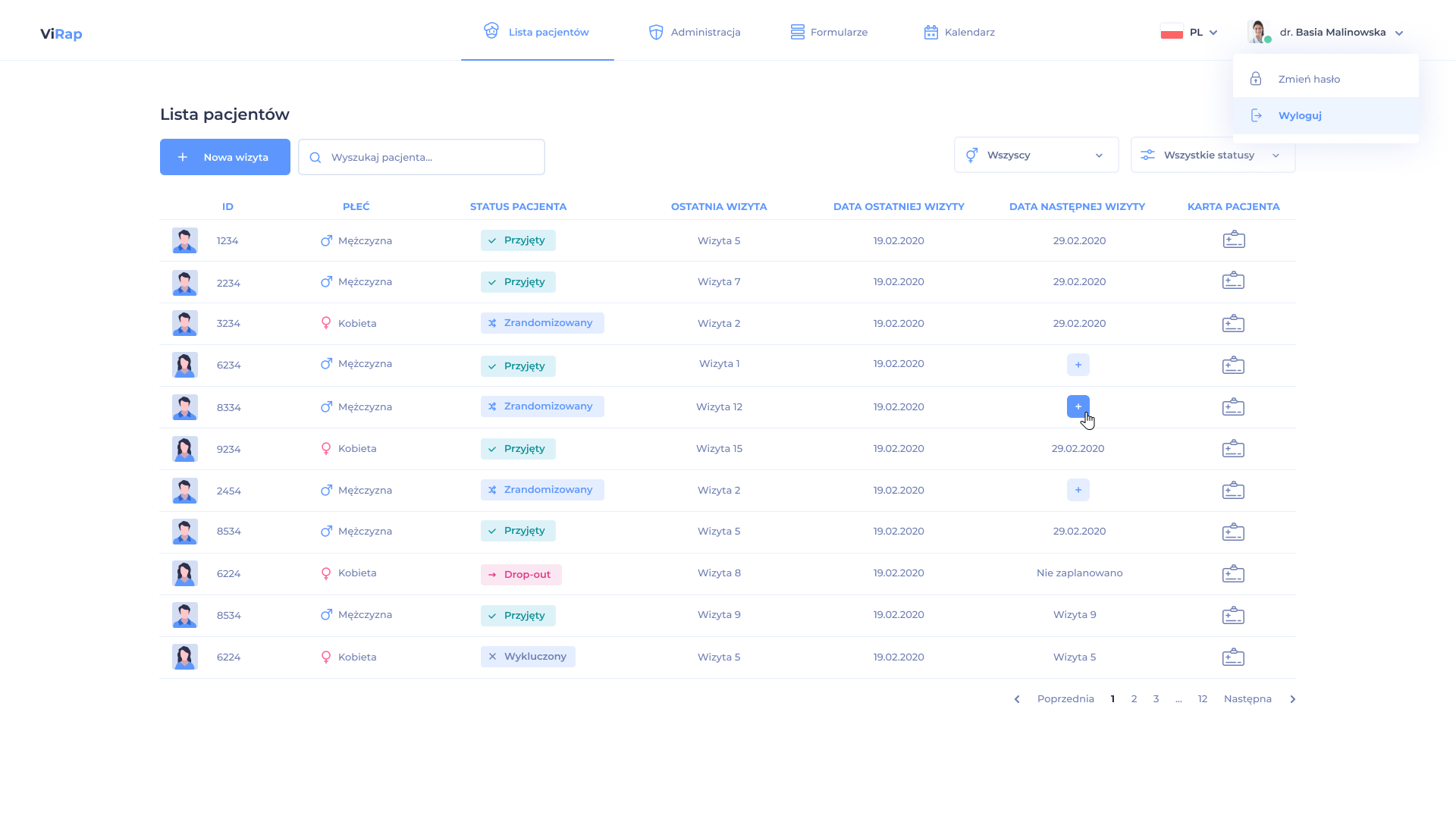
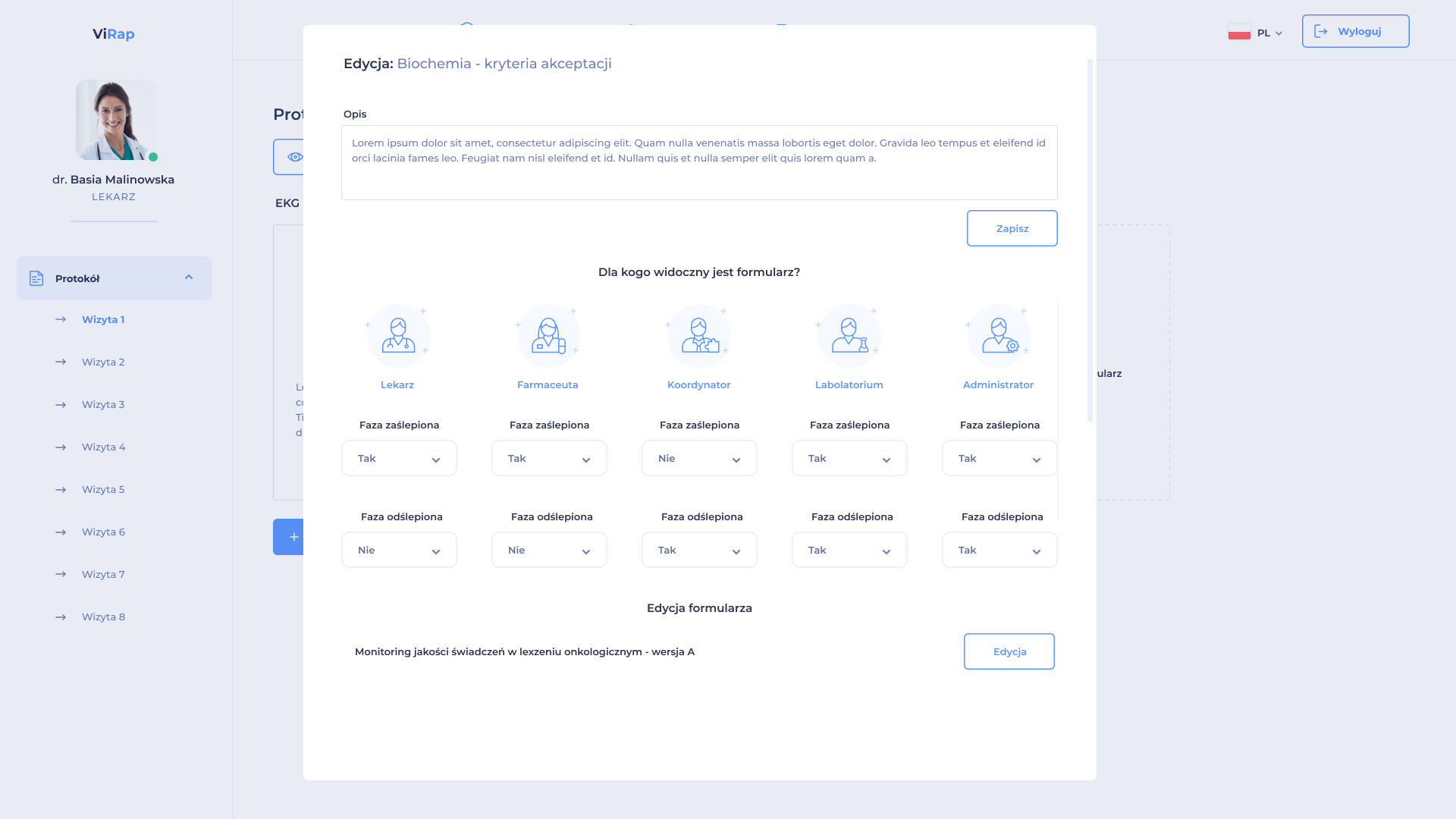
The system enables the creation of a clinical trial in accordance with its protocol and schedule, reflecting the full structure of the course of the clinical trial. It enables the ongoing implementation of modifications resulting from the approved next version of the clinical trial protocol.
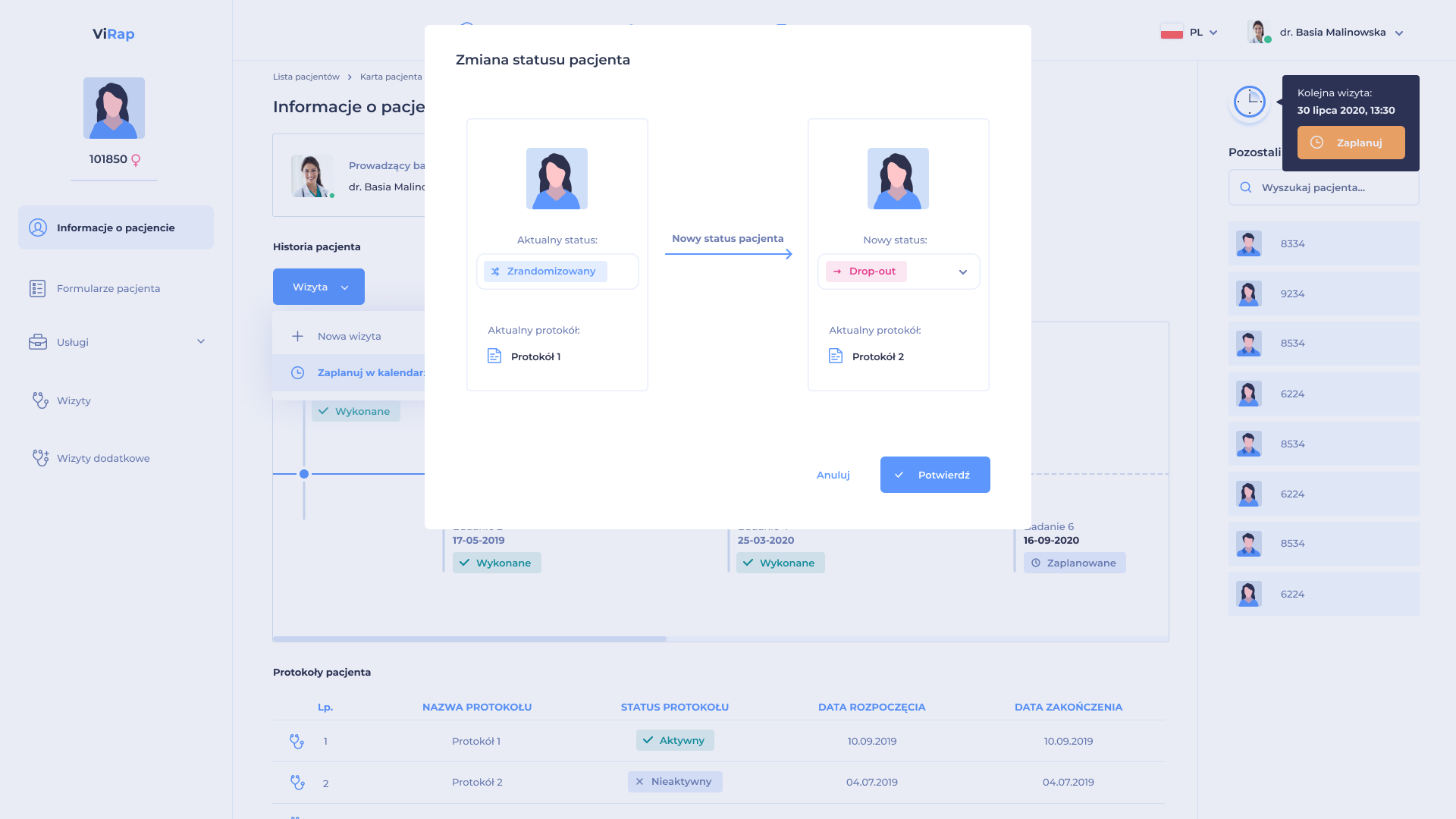
The system enables configuration of randomization in a given clinical trial, taking into account e.g. appropriate randomization method and study arms. The randomization process is carried out in the application automatically by assigning a randomization code based on the criteria specified in the Study Protocol.

eCRF allows you to keep a register of tested products and monitor their condition by notifying the supplier of the demand for delivery of a batch of products, marking defective / disposed of packaging, and alerting the user about the upcoming expiry date of the package. The system enables the dispensing of products to the patient, and in the case of blinded studies, it automatically takes into account the arm to which the patient was assigned during randomization. In addition, it allows you to track the full history of the circulation of a given product.
The eCRF system allows you to keep a register and monitor all stages of processing samples with biological material collected in a given study, which are often sent to an external supplier / laboratory for diagnostics.






Data validation reduces the possibility of entering erroneous information, and also enables the detection of deviations during data entry, by:















www.ttsi.com.pl
sales@ttsi.com.pl
phone: +48 661 900 265
NIP: 527-294-32-09 | REGON: 387666213 | KRS: 0000872108
TRANSITION TECHNOLOGIES SCIENCE Sp. z o.o.